business resources
How To Ensure Product Integrity Of Medical Devices
12 Apr 2023, 11:31 am GMT+1
Medical devices are essential components of the healthcare industry and are integral to the safety and well-being of patients. Therefore, medical device manufacturers must ensure that their products deliver on their promise and maintain the highest levels of product integrity.
There are various reasons for medical device recalls, including product integrity issues. To prevent such occurrences, medical device manufacturers need to take specific steps to ensure the integrity of their products. This article will discuss in detail the measures to ensure the integrity of their products.
1. Consider Working With A Team Of Qualified Manufacturers
The first tip to ensuring the product integrity of medical devices is to work with a team of qualified manufacturers. This team should include professionals from multiple disciplines, such as design, manufacturing, materials, and engineering. This team should be familiar with the current medical device manufacturing regulations and standards and have experience with the specific device in question.
For instance, working with the team behind RemoteM can help ensure the integrity of medical devices that require precise temperature control. Even if you have an in-house team, it is always beneficial to collaborate with external experts to ensure the highest level of expertise and knowledge.
When you visit RBC Medical and other reliable manufacturers, you will learn that working with a team of qualified manufacturers helps ensure the product integrity of medical devices by ensuring that all aspects of the device are considered and adequately addressed.
The team can review the device's design, materials used, and manufacturing processes to guarantee the safety, efficacy, appropriateness, and consistency of the quality of the medical device.
2. Establish A Tracking System
Another way to establish product integrity for medical devices is by establishing a tracking system, such as radio-frequency identification or RFID warehouse tracking. This system uses RFID to track and manage inventory, ensuring the proper devices are delivered at the right time.
RFID warehouse tracking also helps to reduce product loss and even allows for traceability in the case of product recalls. By tracing products back to their source, companies can quickly identify any problems or issues affecting their integrity and take steps to ensure that the product meets the highest standards. This is especially important in the medical device industry, where product integrity is paramount.
3. Use Third-party Auditors To Conduct Regular Inspections And Assessments
While medical device companies may have a rigorous internal quality assurance system in place, it is also vital to have an independent third-party auditor conduct regular inspections and assessments to ensure product integrity. These inspections and reviews can provide an unbiased evaluation of product quality, remind you of vital steps you might have missed, like undergoing medical device licensing processes, and help identify any weaknesses or areas for improvement.
The auditors should be medical device industry experts and understand the relevant regulations and standards. They should use their experience to assess the quality management system and evaluate its implementation. The auditors should verify that the manufacturer follows the appropriate procedures, processes, and protocols for producing medical devices.
During the audit, the product should be inspected. This includes checking for potential risks and reviewing product design and production documents. The audit results should be shared with the relevant stakeholders, including the medical device manufacturer, and corrective actions should be taken.
4. Invest In Technology That Can Prevent Tampering
At a time when tech is taking almost every industry by storm, the medical device industry has been no exception. At a time when tech is taking almost every industry by storm, the medical device industry has been no exception. Investing in state-of-the-art technologies is essential to ensure the product integrity of medical devices and to support broader initiatives such as patient recruitment services, which rely on secure and trustworthy devices to ensure clinical trial success.
One way to ensure product integrity is through tamper-evident packaging. This involves using unique packaging materials, labels, and seals that can detect any signs of tampering, making it hard for unauthorized individuals to gain access to medical devices. For example, some manufacturers use transparent packaging to ensure customers can see the contents and tamper-evident seals or tape to ensure the product has not been tampered with.
Another top technology that can be used to prevent tampering is RFID tagging. RFID tags are embedded with a unique identifier, enabling device tracking throughout its life cycle. These tags can also be linked to security protocols, allowing access only to authorized personnel.
Finally, cryptographic techniques can be used to protect the integrity of medical devices. This includes technologies such as digital signatures, authentication protocols, and encryption techniques which can be used to verify that the device has not been tampered with.
5. Consider Customer Feedback
Like any other sector, customers' response is a great way to measure the integrity of medical devices. By looking into customers' feedback, it becomes easier to identify any issues related to the device, such as performance, accuracy, or reliability. This allows the company to take appropriate action to address any concerns, thereby improving the overall safety and efficacy of the device.
Customer feedback can also provide insight into how the device is used in different medical settings and help the company identify unexpected issues that may go unnoticed. By considering customer feedback, medical device manufacturers can ensure that their products are of the best quality and integrity.
6. Staff Training
Another easy way to ensure the product integrity of medical devices is to train staff on properly using and taking care of the equipment. There are several benefits to this, including the following:
- Proper use of medical devices: This helps ensure the device complies with applicable regulations. This also lets the staff know if the device functions correctly, reducing the risk of breakdowns. Training the team in the proper use of medical devices is necessary to maintain the integrity of the product.
- Safe operation of medical devices: This reduces potential risks associated with the device.
- Maintaining medical devices correctly: This reduces the risk of product failures and malfunctions and improves overall safety and performance.
When your staff is adequately trained, they are more likely to identify and report any issues with a device that could lead to product integrity issues.
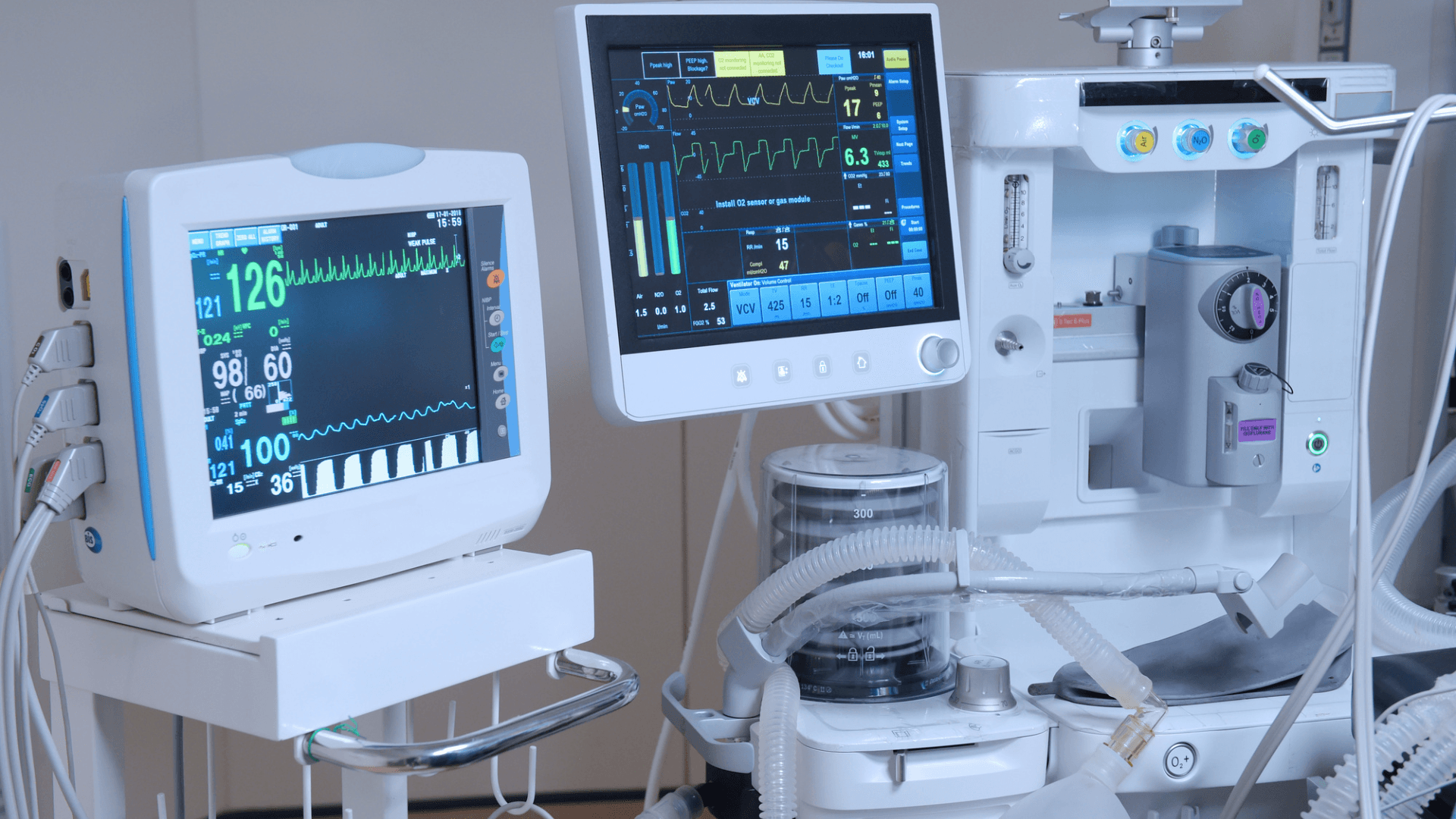
Conclusion
Ensuring the integrity of medical devices is essential for the safety and well-being of patients. Achieving product integrity requires medical device manufacturers to take some measures, such as working with a team of qualified manufacturers, establishing a tracking system, using third-party auditors to conduct regular inspections and assessments, investing in technology that can prevent tampering, and considering customer feedback. By taking these steps, medical device companies can ensure that their products meet the highest safety and efficacy standards.
Share this
Contributor
Staff
The team of expert contributors at Businessabc brings together a diverse range of insights and knowledge from various industries, including 4IR technologies like Artificial Intelligence, Digital Twin, Spatial Computing, Smart Cities, and from various aspects of businesses like policy, governance, cybersecurity, and innovation. Committed to delivering high-quality content, our contributors provide in-depth analysis, thought leadership, and the latest trends to keep our readers informed and ahead of the curve. Whether it's business strategy, technology, or market trends, the Businessabc Contributor team is dedicated to offering valuable perspectives that empower professionals and entrepreneurs alike.
previous
Business Guide: How To Attract New Clients?
next
A Small Business Guide To Cloud Communications